The kinetic properties of salts in a mixture are an area of science in which theoretical calculations deviate from experimental results. To counter this problem the Belgian Science Policy (BELSPO) has funded a four year PhD research, a collaboration between the Royal Institute for Cultural Heritage (KIK-IRPA), Ghent University (Department of Geology), University of Antwerp (Heritage Department), the Belgian Building Research Institute (BBRI), University of Hamburg (UH) and University College London (Institute for Sustainable Heritage).
The research aims to shed light on how environmental conditions influence the formation of complex salts to prevent the deterioration of materials found in the built environment, such as, monuments, archaeological sites, road infrastructures and concrete constructions. There is also an important need to understand the processes of salts in both the academic world and industrial applications. Furthermore, this subject overlaps with problems related to the understanding of geological systems, more specifically concerning chemical weathering and mineral formations on earth and planets.
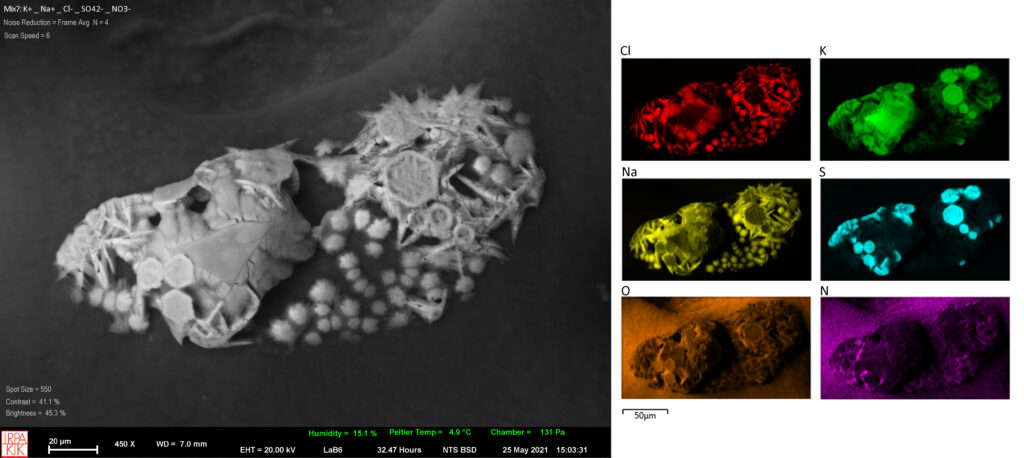
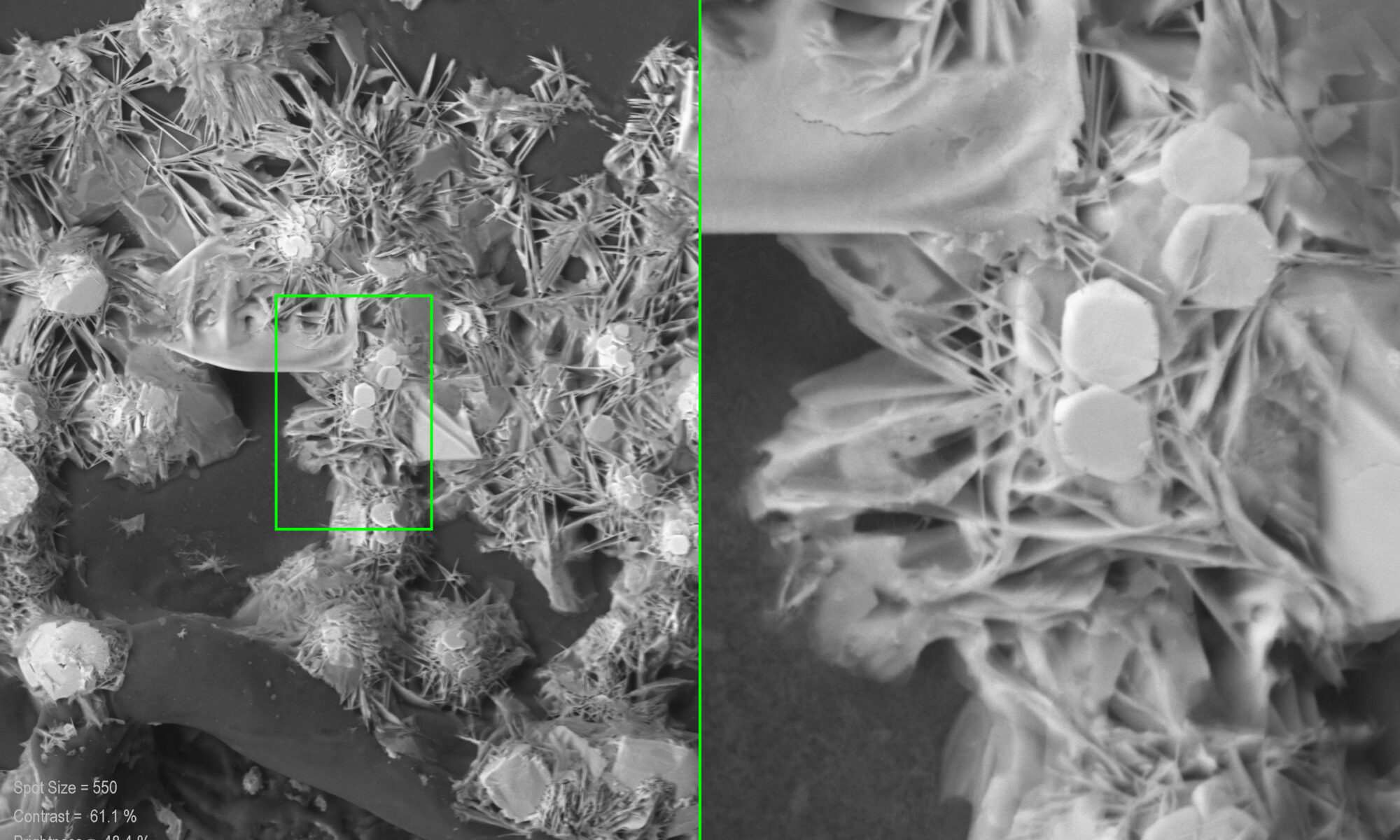
The experiments will focus on salts commonly found in building materials derived from a database containing over 10.000 samples (ion chromatography analysis). The ions under investigation consider a range of mixtures containing Na+, Mg2+, K+, Ca2+, Cl–, NO3– and SO42-. The kinetics of common salt mixtures are studied by means of Environmental Scanning Electron Microscopy/Energy Dispersive X-ray (ESEM/EDX), digital 3D microscopy coupled with a temperature and humidity generator (GenRH/Mcell) and Vapor Sorption Analysis. The phase transitions and the formation of complex salts under realistic climatic conditions will be recorded while salt crystals are identified by means of micro-RAMAN spectroscopy and XRD.
The results are correlated with thermodynamically determined phase transitions of the mixtures (ECOS/RUNSALT) to further aid the development of more accurate computer models. Afterwards, realistic climate data is used to predict the amount of crystallization cycles that occur over time. To validate the findings the research will incorporate case studies, consequently increasing the impact and contribute to the mitigation and understanding of salts. The final goal is to build a solid understanding of salt crystallization in changing climatic conditions and identify specific conditions when salts undergo phase transitions, thus allowing scientist to define critical potential risk events past and future. Project updates and publications can be found here.
For more information contact: Sebastien Godts (sebastiaan.godst@kikirpa.be)