
Epilepsy is one of the most common chronic neurological disorders, affecting more than 65 million people worldwide. I’m Dr. Daniele Bertoglio and I recently obtained my second PhD at UAntwerp under supervision of Prof. Annemie Van Der Linden, head of the Bio-Imaging Lab (BIL). Now, I’ve started working as a postdoctoral fellow at the Molecular Imaging Center Antwerp (MICA). During my PhD I used various imaging techniques to gain a better understanding of epilepsy, a neurological disorder in which brain activity becomes abnormal, causing seizures.
Some types of epilepsy run in families and are both inherited and genetic. Other types of epilepsy can be caused by different types of brain injury, such as status epilepticus (SE), brain trauma, or stroke. Temporal lobe epilepsy (TLE) is the most common form of epilepsy in adults and there are currently no effective treatments. After an initial brain injury, there is a seizure-free period during which several disease-causing changes occur. These include brain inflammation, impairment of cerebral networks, and disruption of synaptic circuitry. Ultimately, these modifications cause the transition of a healthy brain into a hyperexcitable one, leading to the development of epileptic seizures. This whole process that results in epilepsy is known as epileptogenesis.
The seizure-free period that typically occurs between the initial injury and the start of the epileptic seizures is a therapeutic window where medications that could halt or even prevent the onset of seizures could be given. However, the development of medications to stop seizures from occurring remains an extremely ambitious goal and there are still two main challenges that need to be overcome: (1) there is a wide variety of injuries that can trigger the epileptogenic process (such as status epilepticus (SE), brain trauma, or stroke) and (2) we currently cannot accurately predict which people are at risk of developing epilepsy.
During my PhD research at UAntwerp, I worked on identifying specific novel biomarkers in the brain during epileptogenesis to help with detecting patients at risk of developing seizures. In a preclinical rat model of temporal lobe epilepsy, I used non-invasive neuroimaging techniques to assess brain inflammation and brain connectivity. The neuroimaging techniques that were used are positron emission tomography (PET) and magnetic resonance imaging (MRI). PET scans use radioactive substances (known as radiotracers) to visualize changes in metabolic processes, blood flow, and brain receptors in the brain. MRI on the other hand, is a type of scan that uses strong magnetic fields and radio waves to produce highly detailed images of the brain and to detect alterations in brain structure and functional connectivity.
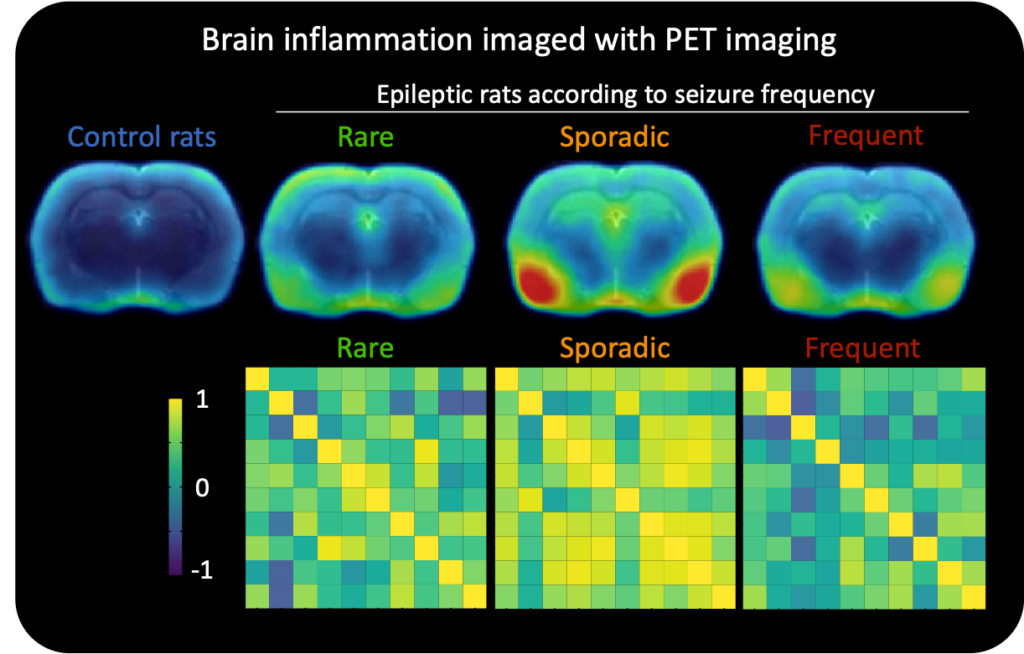
My first step was to figure out which preclinical rat model for epilepsy would be best to use to detect biomarkers. I then did PET scans on these rats to specifically look for brain inflammation at the start of the disease. I found that a single PET scan, taken at the start of the disease, detected brain inflammation and could also accurately predict the seizure frequency of epileptic rats. This suggests that brain scans could potentially be used to identify patients that are at risk of developing seizures.
I also investigated functional brain networks by detecting cerebral activity associated with changes in blood flow during epileptogenesis. As blood releases oxygen at a greater rate in neuronally active areas than inactive ones, the measurement of changes in hemodynamic response can provide information on the activation of specific brain structures and their association. In the rats with epilepsy, we found that there was a disconnect in these functional brain networks at the start of disease and their alteration was related to future disease severity.
During my PhD research, I identified several promising preclinical imaging markers for epileptogenesis that were a good predictor of disease severity. Future studies will be necessary to determine whether these markers could also be used in patients. For my postdoctoral research, I’m now extending the acquired research knowledge on neuroimaging to other neurological diseases, including Huntington’s disease and spinal cord injury.
BIO

Daniele Bertoglio is currently a postdoctoral FWO research fellow at the Molecular Imaging Center Antwerp (MICA), at UAntwerp. He uses various translational neuroimaging tools to quantify structural, functional, and molecular changes in preclinical models of neurological diseases, focusing on Huntington’s disease, temporal lobe epilepsy, and spinal cord injury. His research applies state-of-the-art small animal neuroimaging techniques, including positron emission tomography (PET) and magnetic resonance imaging (MRI), to identify novel candidate biomarkers to monitor disease progression, predict disease outcome, and evaluate the efficacy of innovative therapeutic approaches.
Article written by Dr. Daniele Bertoglio. Edited by Dr. Bronwen Martin.
