New research findings by Prof. Vincent Timmerman’s group have revealed common hallmarks in motor neurons of patients with different subtypes of Charcot-Marie-Tooth disease (CMT) type 2, including impaired axonal transport and mitochondrial dysfunction. Targeting these underlying disease mechanisms could pave the way for treatments that can help a larger group of CMT patients.
The research in Prof. Vincent Timmerman’s lab focusses on understanding the complexity of Charcot-Marie-Tooth disease (CMT), a neuropathy of the peripheral nervous system. CMT progressively affects sensory and motor function causing weakness and atrophy of distal limb muscles, leading to hand and foot deformities. The degree of severity varies between patients, and CMT occurs in both children and adults, affecting 1 in 2500 of individuals. Jonas Van lent, a PhD researcher in Timmerman’s group, used highly specialised induced pluripotent stem cell (iPSC) technology to study motor and sensory neurons that were derived from patients with different subtypes of CMT2 (caused by mutations in the most frequent CMT2 associated genes) and healthy volunteers. Induced pluripotent stem cells are derived from skin or blood cells that have been reprogrammed back into an embryonic-like pluripotent state, which enables the development of an unlimited source of any type of human cell required for therapeutic purposes.
“The genetic diversity of CMT2 causes a particular challenge for the development of treatments, which has led to a scattering of research efforts.”
Jonas: “Each lab is studying their “favourite” gene, which has resulted in the identification of a large number of dysfunctional pathways. The main problem with such gene-centric studies is that it is unclear whether the alterations in these pathways stem from gene-specific defects or impinge on a common pathway that is shared with other causal genes.”
This study addresses these challenges by simultaneously creating iPSC-lines from the most frequent subtypes of CMT2, including two mutations within the same gene. The different CMT2 cell lines were compared to healthy controls and a CRISPR-Cas9 corrected patient line. CRISPR-Cas9 is a unique technology that enables researchers to edit parts of the genome by removing, adding or altering sections of the DNA sequence.
The researchers differentiated the iPSCs to both motor and sensory neurons (the main types of neurons affected by these peripheral neuropathies), and compared multiple CMT2 genes at once. This enabled them to distinguish gene-specific mechanisms from common disease mechanisms.
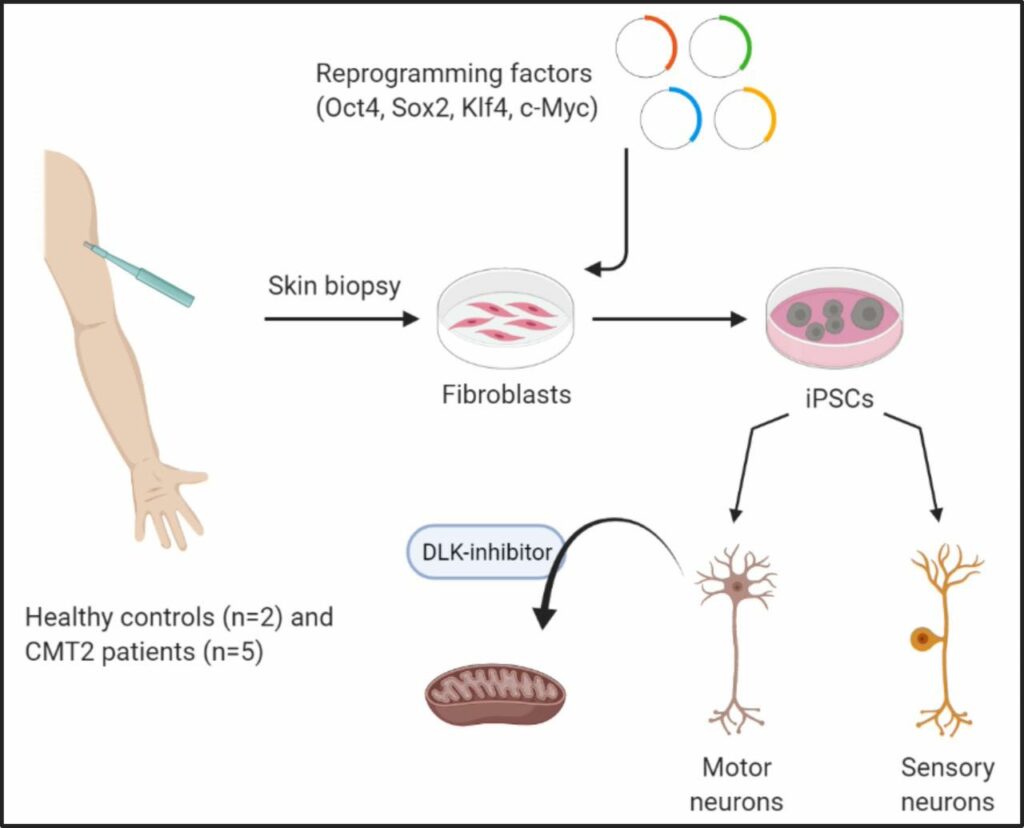
Detailed investigation of these different CMT2 cell lines has led to the identification of common impairments in axonal transport and mitochondrial function. Moreover, the researchers demonstrated that common targets can be identified with this approach, such as the dual leucine zipper kinase (DLK). This could form an important step forward for the identification of new drug targets that would potentially be beneficial for a larger group of CMT patients.
This work was published in BRAIN. You can read the full paper here.
The highlights of this study were also featured in a scientific commentary by Dr. Juliane Müller and Prof. Rita Horvath of the University of Cambridge.
BIO

Jonas Van lent obtained an M.Sc. in Biochemistry & Biotechnology at the University of Antwerp. Currently he is a PhD student in the Peripheral Neuropathy Research Group, where he focusses on CMT. Soon he will be a visiting PhD researcher at the Gladstone Institutes in San Francisco. There, he will further his knowledge and expertise on the underlying disease mechanisms of peripheral neuropathies using iPSC technology.
The Timmerman lab is a member of the UAntwerp µNEURO Research Centre of Excellence and the iMARK valorisation consortium. The lab is supported by the Research Foundation Flanders (FWO), American Muscular Dystrophy Association (MDA), Medical Foundation Queen Elisabeth (GSKE), Association Belge contre les Maladies Neuromusculaires (ABMM), Rotary ‘Hope in Head’ program and EU-H2020 Solve-RD program ‘Solving the unsolved rare diseases’.
Article written by Jonas Van lent, Dr. Liesbeth Vanherp, Prof. Vincent Timmerman. Edited by Dr. Bronwen Martin.
