Dr. Paul Fransen and his group investigated the role of Nitric Oxide (NO) in controlling arterial stiffness. Dr. Paul Fransen, Dr. Arthur Leloup, Mr. Cor Van Hove, PhD student Sofie De Moudt and Prof. Gilles De Keulenaer are members of the Physiopharmacology group. Their research focuses on understanding the role of arterial stiffening in vascular health and aging.
In the circulatory system of the body, the aorta plays the important role of converting the pulsatile blood flow that originates from the beating heart to a nearly-continuous flow in the peripheral vessels that perfuse important organs, such as the brain and kidneys. During aging, the aorta becomes progressively stiffer. As a result, the aorta does not efficiently dampen the pulsatile blood flow and this typically coincides with arterial hypertension. The prevalence of arterial hypertension averages 70% in the age range above 65 years (Heart and Stroke Statistics of the American Heart Association, update 2014) and even after decades of research on the mechanisms of hypertension, current treatment strategies are not always effective at reducing blood pressure or reducing cardiovascular disease.
Interestingly, the REASON study reported in 2009 that a poor response to antihypertensive treatment is linked to stiffness of large arteries. In addition, several studies have shown that arterial stiffness is a better predictor of cardiovascular risk than hypertension as such. Hence, “the chicken and the egg” discussion whether arterial hypertension leads to arterial stiffening or the other way around has become the research focus for many clinicians and researchers. Research by the Physiopharmacology group and other groups, in which both blood pressure and vascular stiffness were measured in a longitudinal manner, provide evidence that arterial stiffness precedes hypertension and cardiac dysfunction. Hence, arterial stiffening may be the etiological factor of arterial hypertension, rather than an adaptive response of the vessel wall to restore wall tension according to Laplace’s law.
As the mechanisms that regulate the stiffness of the aortic wall are still incompletely understood, a better understanding of these mechanisms is an important step towards more effective treatments for age-related arterial stiffness, hypertension and associated complications.
The main hypothesis of the Physiopharmacology group is that dysfunction of active components of the vessel wall, i.e. the endothelium and vascular smooth muscle cells, contribute to stiffening of the arterial system, especially in the earliest stages of disease. This hypothesis is supported by the observations that, in addition to increased arterial stiffness, endothelial dysfunction is a predictor of incident hypertension and a hallmark of vascular aging. Consistently, in both isolated aortas and animal models of endothelial dysfunction, reduced bioavailability of NO, a relaxing factor released by the endothelium, induces arterial stiffness, vascular wall remodeling and hypertension. Hence, mouse models with altered NO signaling could be good models for investigating the (patho)physiological role of NO signaling as a dynamic regulator of arterial stiffness.
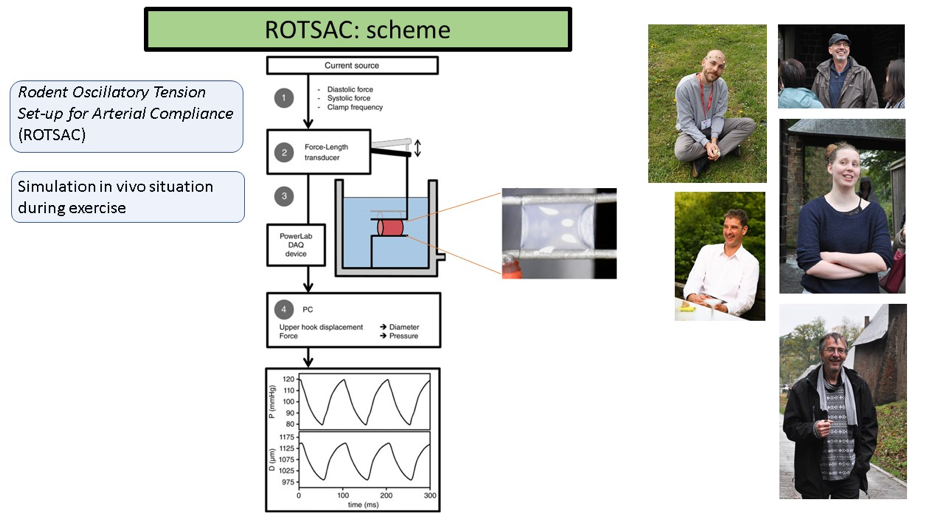
In American Journal of Physiology Fransen’s group recently published a study, in which they characterized the ex vivo biomechanical properties of aortic segments from mice with no (eNOS-/-), normal (WT) or high (eNOS-tg) endothelial NO synthase (eNOS) expression. They found that endothelial function and NO bioavailability are important determinants of aortic biomechanics. Therefore, they developed a new technique, named “Rodent Oscillatory Tension Set-up for Arterial Compliance (ROTSAC)”, to investigate the biomechanical properties of isolated aortic segments, while exposing them to different pharmacological and mechanical stimuli. Fransen’s group compared the dimensions and stiffness parameters of the isolated aortic segments from the different mouse models with different eNOS expression levels under similar conditions of simulated transmural pressure and stretch frequencies that closely mimic in vivo conditions. The diameter and compliance were found to be lower in eNOS-/- mice and increased in eNOS-tg mice, compared to WT mice.
Interestingly, these differences remained when NO levels were pharmacologically restored ex vivo by supplying a NO donor, suggesting that they were not merely the result of a lack or excess of the vasodilator effects of NO. Analysis of basal vascular smooth muscle cell tone and the phasic, as well as the tonic contraction, in response to α1-adrenergic stimulation with phenylephrine revealed that the chronic lack of eNOS expression affected aortic reactivity similarly, but with a different magnitude as compared to acute eNOS blockade using L-NAME in WT and eNOS-tg mice. These experiments clearly show that chronic distortion of NO signaling triggered several compensatory mechanisms that reflect the organism’s attempt to maintain optimal central hemodynamics. The clinical relevance of these data is that acute and chronic endothelial dysfunction affects aortic compliance and that chronic distortion of these pathways triggers compensatory mechanisms, further supporting the hypothesis that NO-related pathways play an important physiological role in the aorta.
Given the clinical relevance of arterial stiffness as a predictor of cardiovascular risk, NO-signaling pathways could become an attractive pharmacologic target when the underlying mechanisms of arterial stiffening are fully unraveled.
Article written by Dr. Paul Fransen and edited by Dr. Bronwen Martin.
