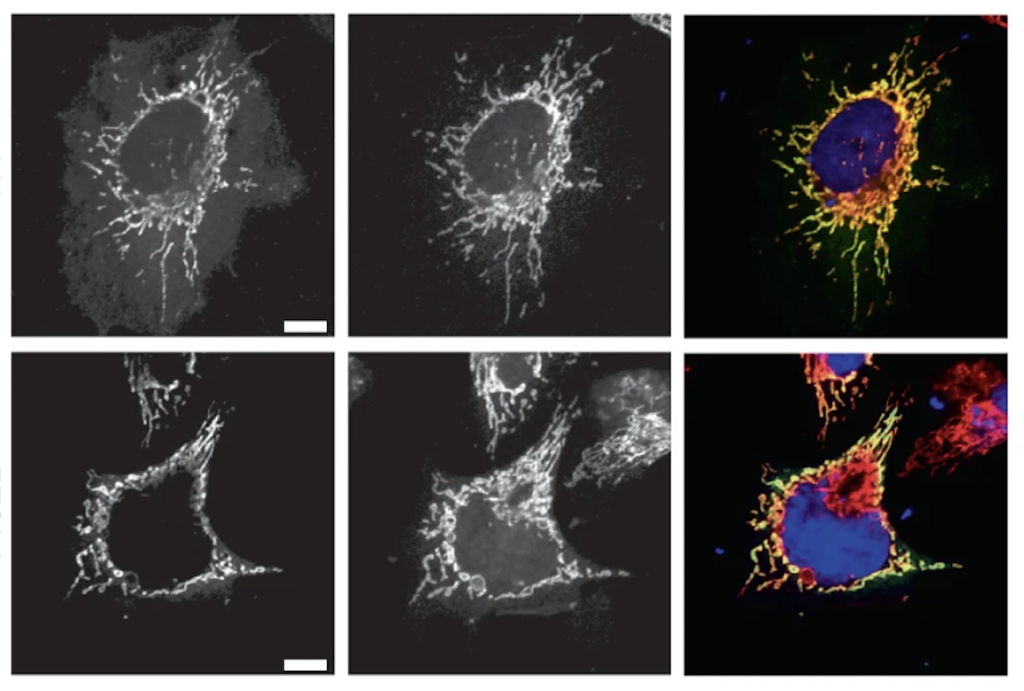
CMT disease-causing mutations in HSPB1 disturb its mitochondrial function.
Adapted figure from Nat Cell Biol. 2023 Jan 23.
In a recent study published in the journal Nature Cell Biology, Dr. Elias Adriaenssens, Prof. Vincent Timmerman and colleagues have discovered a new function for a group of proteins called small heat shock proteins (HSPBs) in the human body. These proteins, which are found in the liquid (cytosolic) portion of cells, were found to play a critical role in the maintenance of the powerhouses of cells, the mitochondria.
The researchers discovered that HSPBs actually travel to a specific location within the mitochondria, known as the intermembrane space. Once there, these proteins act as “molecular chaperones” – meaning they help to fold and maintain the proper shape of other proteins within the mitochondria. This process is crucial for the overall health and function of the mitochondria, which are responsible for producing the energy that our cells need to function properly.
This study also found that mutations in the HSPBs genes can lead to defects in this protein chaperoning function, which could be a contributing factor to a nerve disorder called Charcot-Marie-Tooth (CMT) neuropathy. CMT is a genetic disorder that affects the nerves in the feet, legs, and hands, leading to muscle weakness and atrophy. This research suggests that the malfunction of HSPBs within the mitochondria may play a role in the development of CMT.
In summary, this study has shed light on the new role of small heat shock proteins in the maintenance of the health of mitochondria, and how defects in these proteins can lead to the development of certain diseases.
Written by Dr. Bronwen Martin & Dr. Liesbeth Vanherp.
