I’m Gaëlle Houthaeve and for the past 5 years I have been doing an interdisciplinary PhD with Prof. Winnok De Vos in the Laboratory of Cell Biology and Histology and Prof. Kevin Braeckmans (Pharmaceutical Sciences, Ghent University). My PhD research has focused on vapor nanobubble photoporation, which uses laser light (“photo”) to generate vapor nanobubbles, which are able to porate the plasma membrane of cells (“poration”).
Various applications demand delivery of specific molecules into the cytosol. An example is cell-based therapy, in which patient-derived cells are genetically modified and re-introduced into the patient to treat disease.
Vapor nanobubble photoporation has already been proven to be efficient for permeabilization of the plasma membrane of a wide range of cell types, with minimal toxicity. Despite its promise, however, still little is known about the effects of vapor nanobubble photoporation on the cell’s well-being.
First, gold nanoparticles are allowed to attach to the plasma membrane. Subsequently, cells are irradiated with high intensity laser pulses. This causes the gold nanoparticles to rapidly heat up, which results in the generation of vapor nanobubbles in the surrounding medium. These vapor nanobubbles rapidly expand and collapse, which causes disruption of the plasma membrane, hereby allowing introduction of molecules into the cell.
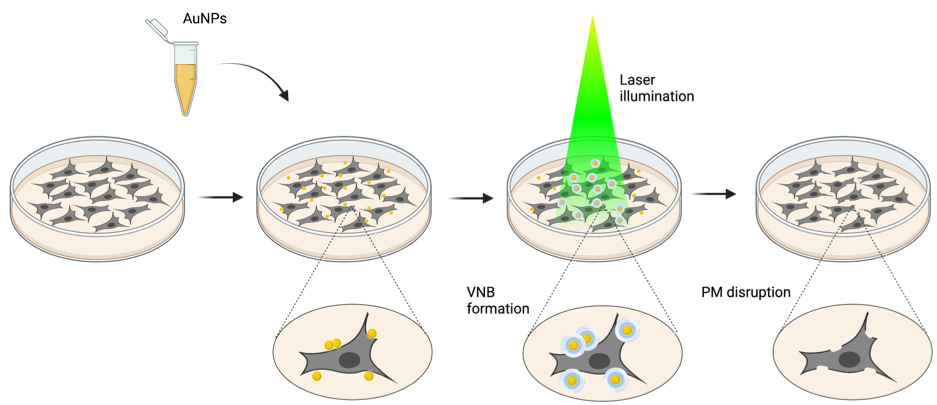
Elucidation of the cellular response to vapor nanobubble photoporation-induced plasma membrane disruption could help to diminish detrimental side effects and thereby increase efficiency of intracellular delivery. Thus, we set out to investigate what impact vapor nanobubble photoporation has on a cell.
We found that in response to vapor nanobubble photoporation-mediated disruption of the plasma membrane, a stiffening of the cell’s nucleus occurs. We found this to be a protective response, as blocking nuclear stiffening caused the cells to have more difficulties to recover. Moreover, via artificially increasing nuclear stiffness, cells were better able to cope with the consequences of vapor nanobubble photoporation. This shows that knowledge on the cellular response to photoporation can be exploited to increase its efficiency, which will be vital for the technique to compete with other methods that allow intracellular delivery.
In some applications, cytosolic delivery is not sufficient and introduction into the cell nucleus is required. This requires disrupting the nuclear envelope, the barrier that surrounds the nucleus. Controlled disruption of the nuclear envelope could also aid with studying the consequences of nuclear envelope ruptures. These events have been found to occur in a group of genetic diseases that are characterized by a vulnerable nuclear envelope.
Up until now, detailed investigation of nuclear envelope rupture effects has been difficult because it is impossible to predict when and where they will occur. Thus, a method that allows the production of nuclear envelope ruptures, with specific control over the timing and location of the ruptures, would enable us to study their consequences in more detail.
Therefore, in the second part of my PhD, I investigated the potential of vapor nanobubble photoporation for the disruption of the nuclear envelope. We found that vapor nanobubble photoporation is able to induce nuclear envelope ruptures that mimicked the spontaneous nuclear envelope ruptures to a high degree. We also found that nuclear envelope photoporation allows delivery of molecules into the nucleus interior. Once fully optimized, nuclear envelope photoporation will not only be a substantial asset for research on nuclear envelope ruptures, it will also become a valuable tool for applications that require the transient and controlled disruption of the nuclear envelope.
A portable device that allows vapor nanobubble photoporation has recently been commercialized by a UGent spin-off company called Trince.
Further reading:
G. Houthaeve, R. Xiong, J. Robijns, B. Luyckx, Y. Beulque, T. Brans, C. Campsteijn, S. Samal, S. Stremersch, S. De Smedt, K. Braeckmans and W. De Vos (2018). Targeted Perturbation of Nuclear Envelope Integrity with Vapor Nanobubble-Mediated Photoporation. ACS Nano 12 (8), p. 7791-7802. Open Access version
Article written by Gaëlle Houthaeve. Edited by Dr. Bronwen Martin.
