
I’m Lorenzo Cianni and for the past year I’ve been working as a Postdoctoral researcher with Prof. Pieter Van Der Veken in the Medicinal Chemistry group (UAMC). Before arriving in Belgium, my journey as a chemist started in Italy, where I obtained my Bachelor’s degree from the University of Rome, La Sapienza. After that I moved to Portugal (University of Porto) and China (Sun Yat-Sen University) to obtain my Master’s degree. Then, I crossed the Atlantic Ocean several times to do my PhD at the University of Sao Paulo in Brazil, the University of Bonn and the University of Tübingen in Germany. After completing my PhD, I worked for an R&D company (Selvita) in Poland for one year before starting at the University of Antwerp. So as you can see, my research has taken me all over the world!
Currently, my research is part of the ATLANTIS research consortium (AuTophagy in InfLAmmatioN and inflammaTory dISorders) which brings together a team of expert researchers from complementary research fields at different universities (UAntwerpen, KULeuven, and UGent).
The ATLANTIS program brings together the burgeoning fields of cell death/inflammation, autophagy and vascular biology. Autophagy (or ‘self-devouring’) is the body’s way of cleaning out damaged cells, in order to regenerate newer, healthier cells. It is a highly evolutionarily conserved cellular process and defects in autophagy have been linked to various diseases, such as neurodegeneration and cancer.
Over the past years, the fields of inflammation-dependent cell death and autophagy have grown tremendously due to the increased recognition of the role played by pathological or therapy-induced cancer cell death. Autophagy has also been shown to play a key role in inflammatory/immune responses and in vascular-protective processes. However, major challenges in treating inflammatory diseases remain due to our limited understanding of how dysregulation of vasculature-associated inflammatory pathways affects tissue and organ homeostasis.
Several medications that modulate autophagy have been developed, suggesting that the autophagic machinery can be manipulated to treat human diseases. However, these medications, among which the autophagy-inducer rapamycin or autophagy-inhibitor hydroxychloroquine/chloroquine, lack specificity, and have moderate potency and substantial toxicity. Another level of complexity comes from the differential effects that autophagy may have, which can either support or impede the biological functions of distinct cell types.
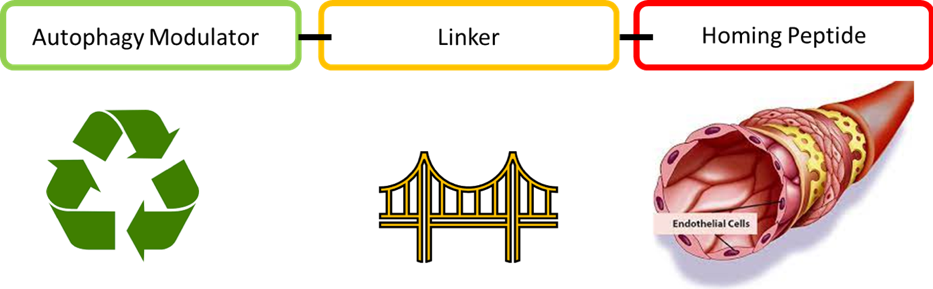
My task is to develop tissue-targeted specific autophagy modulators using medicinal chemistry. One approach that I use is the ‘vascular ZIP-coding’ approach. This is a method for targeting compounds to a specific tissue of interest. It consists of chemically linking a homing peptide to a specific molecule of interest, which has been shown to selectively bind to the tumour vasculature. In this way, these compounds will not have systemic exposure. Therefore, they should have significantly fewer side effects and a higher potential for drug development.
I’ll be further building on this research by also focusing on targeting autophagy in cardiovascular and metabolic diseases. I was awarded a Marie Skłodowska-Curie Actions (MSCA) Postdoctoral grant for this specific project. I’ll be working together with Prof. Pieter Van Der Veken, Prof. Wim Martinet and PhD candidate Michelle Zurek to tackle tissue-specific induction of autophagy as an innovative therapeutic strategy for cardiovascular and metabolic diseases.


Dr. Lorenzo Cianni working in the lab on developing new autophagy modulators. (Photographs taken by Nicolò Filippi).
Western-style diets are hypercaloric and characterized by high fat and high sugar content. Consequently, they are responsible for an epidemic of cardiovascular disease, including atherosclerosis and metabolic disorders, including Non-Alcoholic Fatty Liver Disease. The European population is becoming increasingly exposed to these disorders, for which the only available therapeutic option is lifestyle modification. This typically involves dietary changes and physical activity, but patient compliance with these measures tends to be suboptimal. Pharmacological treatment options could therefore have significant potential for improving patient health.
My research project aims to synthesize specific compounds that can avoid systemic exposure by targeting them specifically to disease-relevant tissues. First, potent autophagy inducers will be chemically linked to selected ‘homing peptides’, and then we aim to specifically deliver these compounds to dysfunctional vascular endothelial cells in atherosclerosis. We will also use a similar approach for Non-Alcoholic Fatty Liver Disease.
My MSCA-funded project has just started this summer and will last for two years. We aim to accomplish the first proof-of-concept for tissue-specific induction of autophagy as an innovative therapeutic strategy for cardiovascular and metabolic disease. So stay tuned…
Article written by Dr. Lorenzo Cianni. Edited by Dr. Bronwen Martin.
