Two patients with amyotrophic lateral sclerosis (ALS) may share the same symptoms, yet their brains may be fighting entirely different battles. Prof. Philip Van Damme, neurologist at UZ Leuven, and Prof. Renzo Mancuso of the VIB-UAntwerp Center for Molecular Neurology uncovered fundamental differences in the brain’s immune response between inherited and non-inherited forms of ALS.
ALS is a deadly disease affecting approx. 1 in 400 adults. Patients are confronted with a gradually increasing loss of the neural cells that control the muscles, resulting in loss of strength that rapidly spreads and worsens. Most patients die within a few years of first symptom onset.
Same symptoms, different biology
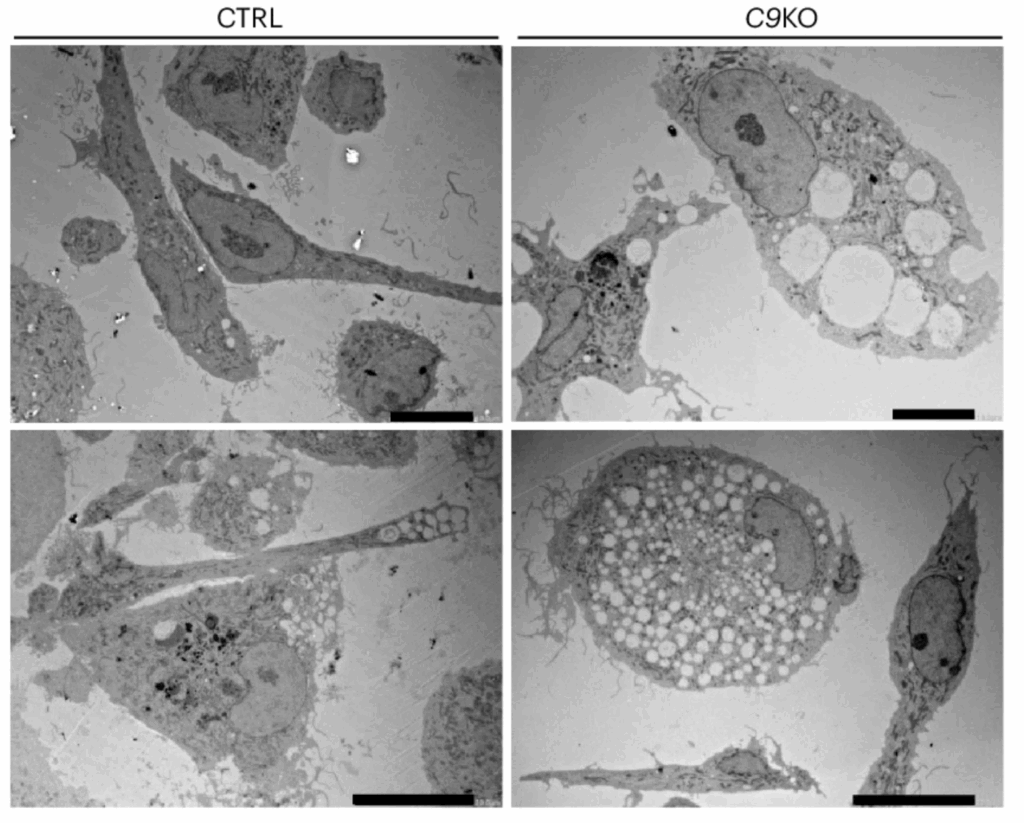
Together, Van Damme, Mancuso and their team members set out to map cell-by-cell changes in both the brain and the spinal cord of patients having the most common genetic mutation predisposing them to ALS, as well as patients with no known genetic link (so-called sporadic patients).
Although inherited ALS and sporadic ALS may look the same at the bedside, their cellular and molecular substrates differ, with direct implications for patient stratification and therapy selection.

Center for Molecular Neurology
To better map the specific disease mechanisms underlying different forms of ALS, Prof. Van Damme and Prof. Mancuso’s teams used patient samples and stem cell systems to study human immune and support cells, called microglia and astrocytes, respectively.
‘We found that microglia, the brain’s immune cells, behave differently in sporadic versus genetic forms of ALS’, explains Van Damme. ‘While microglia clearly switch on into an active, “damage-responding” state, in the case of sporadic ALS, the same microglia were less able to make this switch in the genetic cases under investigation.’

Prof. Philip Van Damme,
neurologist at UZ Leuven
‘Astrocytes were also affected’, adds Mancuso. Astrocytes from the inherited form of ALS displayed a dampened response compared to the sporadic ALS cases. ‘The evidence points towards impaired communication between microglia and astrocytes.’
A milestone for and thanks to patients
‘This is the first integrated, side-by-side comparison of microglia and astrocyte states in genetic versus sporadic forms of ALS’, says Mancuso. ‘Our findings indicate that one-size-fits-all immunomodulatory approaches are unlikely to work in ALS.’
This research was only possible because of the generosity of patients and families who donated brain and spinal cord tissue, says Van Damme. ‘With today’s single-cell technologies, we can study ALS mechanisms in extraordinary detail in human tissue, and I’m convinced this will accelerate progress in the coming years.’, Prof. Van Damme concludes.
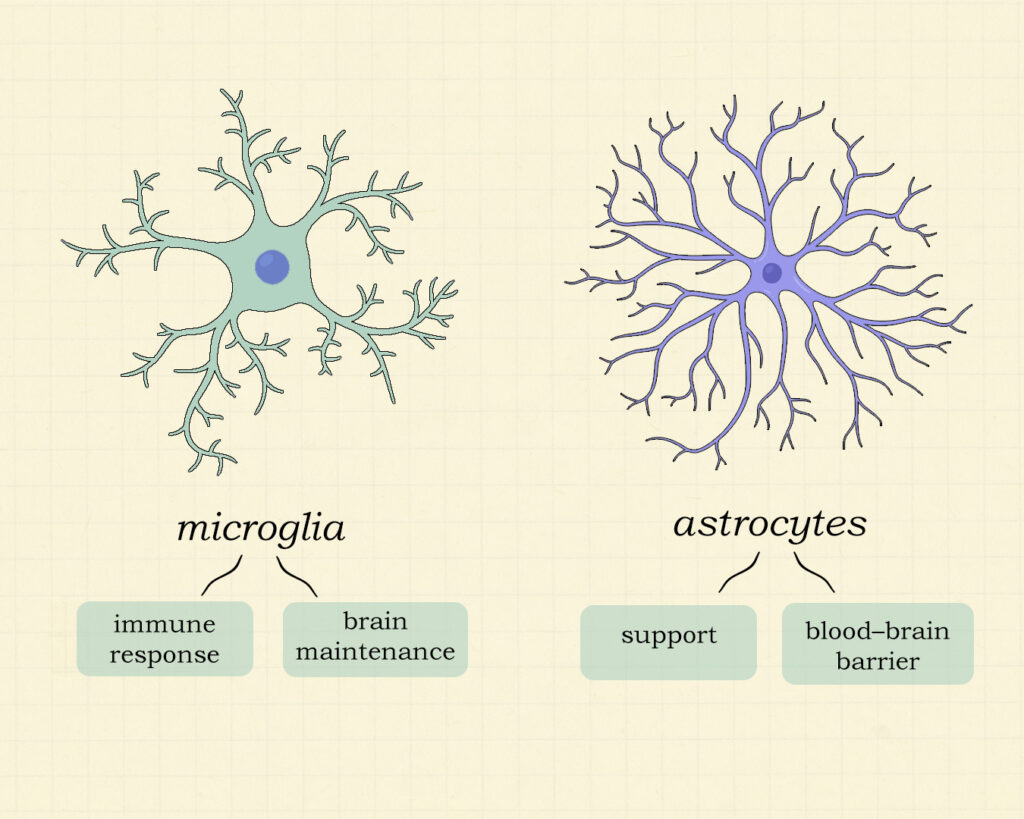
Microglia
Are the immune cells of the brain. They constantly monitor their surroundings for signs of damage, plaques or infection. Microglia are key cells in brain maintenance.
Astrocytes
Are star-shaped cells in the brain and spinal cord that support neurons by regulating the blood–brain barrier, supplying nutrients, maintaining ion and neurotransmitter balance and respond to injury by releasing signals that influence inflammation and repair.
The work was funded by the ALS Liga, ERC, FWO, KU Leuven (a.o. Opening the Future fund and ALS funds), BrightFocus Foundation, SAO, and VIB.
Their research is published in Nature Neuroscience 2025.

